Zelrix electronic anti-migraine patch heads to the FDA for review
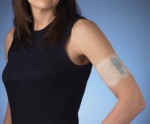
We’ve seen some electronic devices that promise to cure migraines in the past, but NuPathe’s Zelrix patch certainly seems to be among the most practical, and it’s just gotten one step closer to the US market. The company recently announced that the FDA has accepted its filing for a New Drug Application, and it says it now has a target date of August 29, 2011 for the FDA to complete its review. As for the patch itself, it’s a single-use patch that relies on a mild electrical current to “actively transport” the anti-migraine drug sumatriptan through the skin using a process called iontophoresis. That, NuPathe says, not only allows for a more consistent and controlled delivery of the drug, but it also circumvents the nausea and vomiting that can occur when taking the medication orally — which the company notes can be enough to cause some folks to avoid taking the medication altogether. Head on past the break for the complete press release. Continue reading Zelrix electronic anti-migraine patch heads to the FDA for review Zelrix electronic anti-migraine patch heads to the FDA for review originally appeared on Engadget on Sun, 23 Jan 2011 23:22:00 EDT. Please see our terms for use of feeds . Permalink


Posted by
on January 24, 2011. Filed under
News,
Tech.
You can follow any responses to this entry through the
RSS 2.0.
You can leave a response or trackback to this entry

